Supercritical Fluid Extraction of Lithium from a Range of Sources
Researcher: Arna Pálsdóttir
Principal Investigator: Professor Jefferson Tester
Rechargable lithium-ion batteries are widely used for energy storage. The applications are in areas ranging from portable electronics to batteries for electric vehicles. Current methods for lithium mining are focused on highly concentrated brines but with increased use of lithium we will have to move on to less concentrated brines in the future. Our plan is to develop a pre-concentration method for lower concentration brines.
Successful supercritical fluid extraction (SFE) of metals was first accomplished with copper in the 1990s. A supercritical fluid is a substance at conditions above the critical point. The characteristics of supercritical fluids are a mixture of liquid and gas characteristics that can be tuned by small alterations in pressure and temperature. The advantage of using supercritical fluids for extraction is enhanced mass transfer across the interface between phases since the supercritical fluid has low viscosity, high diffusivity and low surface tension. The enhanced mass transfer is important for low concentration brines. The supercritical fluid we use is carbon dioxide (CO2). CO2 is in many ways the best supercritical fluid for this application. The critical point of CO2 is moderate, at 31.1°C and 72.9 atm, CO2 is non-flammable and it does not degrade and is therefore ideal for a closed loop extraction process. SFE of lithium has not been performed before so besides the application there will be a theoretical component. Experiments will be performed to determine the best chelating agent for the extraction process, along with different modifiers and surfactants. The theoretical component will be the determination of mass transfer coefficients and kinetic constants for the system with simple computer modeling. We are currently focusing on the pre-concentration of lithium from geothermal brines but future plans involve the pre-concentration of lithium from non-geothermal brines and seawater.
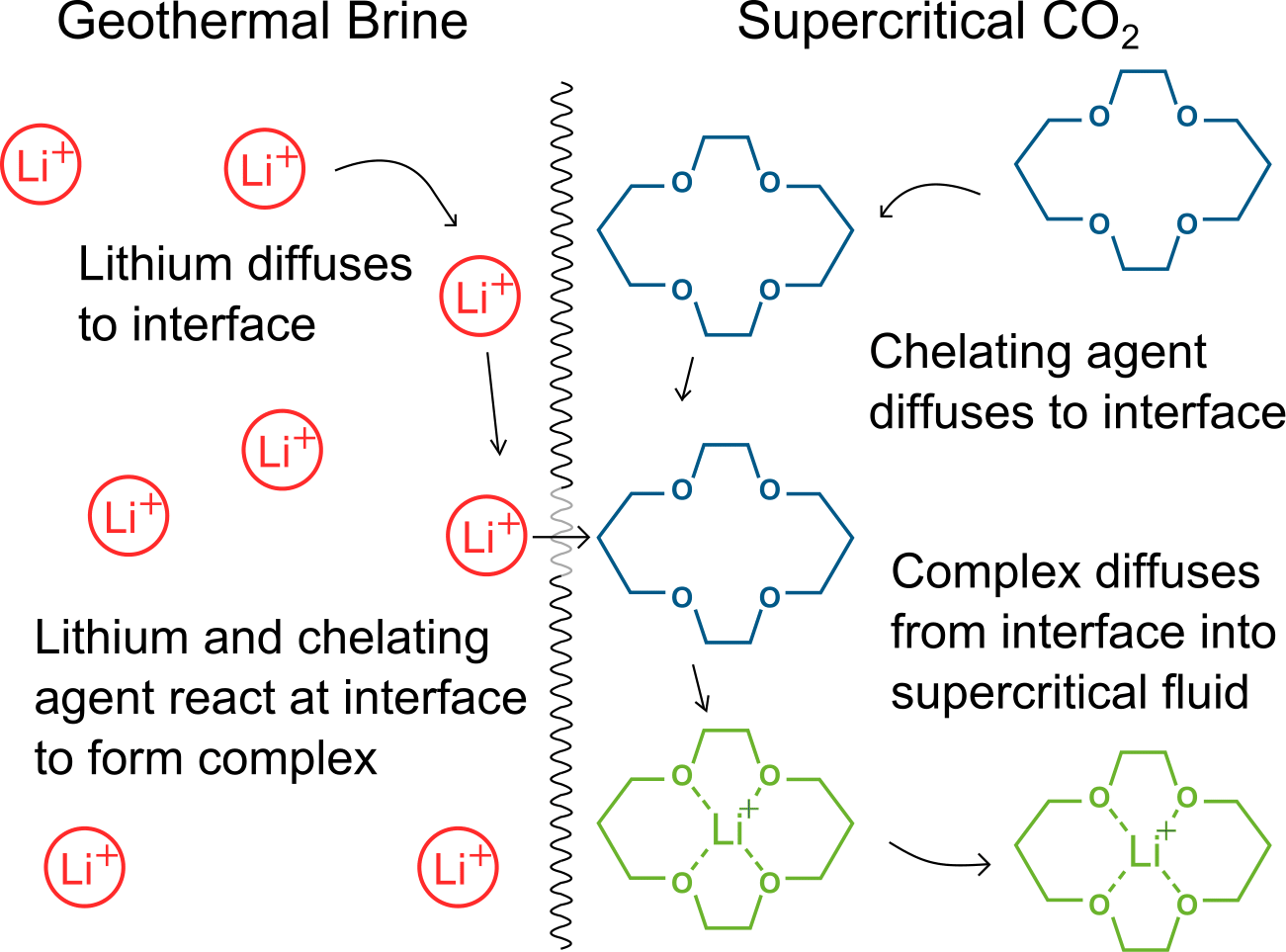
A depiction of lithium extraction from an aqueous solution with a supercritical fluid. The chelation reaction happens at the interface and the resulting complex is immiscible with the supercritical fluid. The mass transfer across the interface is facilitated by the low viscosity, high diffusivity and low surface tension of the supercritical fluid.
