Monte Carlo Molecular Simulations to Determine the Isobaric Heat Capacity of Supercritical Fluids for Thermal Energy Storage Applications
Researcher: Lauren Stutzman
Principal Investigator: Professor Jefferson Tester
In the vicinity of their critical point, supercritical fluids experience enhancements in isobaric heat capacity. Few measurements have been taken of this phenomenon, especially near the gas-liquid and liquid-liquid critical loci of mixtures. Changes in mixture composition can yield different locations of isobaric heat capacity enhancements in pressure and temperature space, suggesting that a mixture of supercritical fluids could be tailored to applications in thermal energy storage, which require fluids to have high energy storage density in specific temperature ranges.
Supercritical fluid mixtures could serve as alternative thermal energy storage mediums to molten salts, which freeze at low temperature, and phase change materials, which cannot be pumped. My research currently focuses on using Monte Carlo molecular simulations to determine enhancements in the isobaric heat capacity of fluids in the vicinity of the critical point. The results of these simulations will help determine which fluids are ideal for thermal energy storage, and elucidate thermodynamic phenomena that very few researchers have explored.
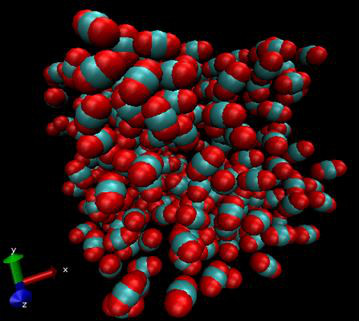
This is a snapshot of a Monte Carlo molecular simulation of supercritical carbon dioxide held at pressure 22.8 MPa and temperature 120 ºC. Molecular simulations compute thermodynamic properties of systems from interactions between different configurations of molecules in small systems (200 to 500 molecules).
